
Since the molecules are flying around in the void most of the time, any increase in the contact they have with one another will increase the intermolecular force which will ultimately lead to a disability for the whole substance to move. The viscosity of a gas, however, increases as temperature increases because there is an increase in frequency of intermolecular collisions at higher temperatures. Why is this the case? As temperature increases, the average speed of molecules in a liquid also increases and as a result, they spend less time with their "neighbors." Therefore, as temperature increases, the average intermolecular forces decrease and the molecules are able to interact without being "weighed down" by one another. It is also important to note that the viscosity of liquids and gases are affected by temperature but in opposite ways meaning that upon heating, the viscosity of a liquid decreases rapidly, whereas gases flow more sluggishly. Because part of a fluid moves, it forces other adjacent parts of the liquid to move along with it causing an internal friction between the molecules which ultimately leads to a reduced rate of flow.

One may ask the question of what is actually going on in the liquids to make one type flow faster and the other more resistant to flow such as the comparison between honey and water earlier. The opposite of viscosity is fluidity which measures the ease of flow while liquids such as motor oil or honey which are “sluggish” and high in viscosity are known as viscous.
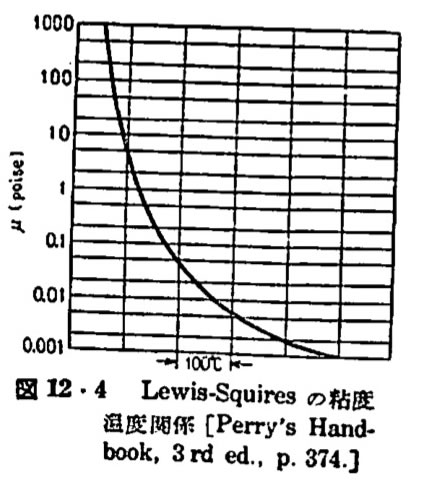
Viscosity can be not only a fluid’s resistance to flow but also a gas’ resistance to flow, change shape or movement.


 0 kommentar(er)
0 kommentar(er)
